Stay Awesome

Keeping up to date: Things to know in practice currently:
19th Jan 2026: Updated versions of the GP and community pharmacy seasonal vaccination service specifications have been published. These have been updated to reflect changes to the COVID-19 vaccine ordering process, and new terminology for patients who may require a COVID-19 vaccination outside of usual campaign timing.
19th Dec: COVID-19 Green Book Chapter updated. (Very small change of citation)
15th Dec 2025: Service specification is out for community pharmacy seasonal vaccination services – COVID-19 and influenza vaccination advanced services. For the first time the COVID-19 and adult influenza vaccination service specifications have been combined into one document, although contractors can still sign up to deliver adult influenza vaccinations only. Also JCVI advice has been accepted on spring 2026 COVID-19 vaccination programme. Basically - it's the same eligibility as 2025.
20th October: Well this is an intriguing title: COVID mRNA vaccines may be able to train immune system to attack cancer cells, boost survival
20th Oct: A reminder of some useful public facing resources you can order for FREE for COVID-19 - leaflets and stickers.
13th October 2025: The SIREN study has been investigating SARS-CoV-2 infections and acute respiratory illness in healthcare workers since 2020, and providing vital research into the immune response to infection and vaccination. New published papers have been added.
11th October: There has been some confusion about the eligibility changes, particularly among people that can get the flu vaccine but may not be able to get the COVID-19 vaccine this year. The following has been done in response:
National flu booking service (NBS) wording has been updated so it is clearer that some people eligible for the flu vaccine may not be able to also get the COVID-19 vaccination due to the eligibility changes
Direct communications have been sent to people under 75 with NBS booked COVID-19 appointments to check their eligibility
Changes are being made to future national direct communications
Communication materials are available including posters, digital display screens, social media materials and an explainer document via the Campaign Resource Centre.
7th October: VERY topical on imms updates at the moment is this issue: Pharmacies facing angry patients over Covid jab confusion. Similar anecdotal reports are coming from GP surgeries too which I get to hear all about on the courses... Sounds like there is improved messaging as a consequence now about who is eligible. We certainly don't want to make anyone any angrier about vaccines!!
16th September: The COVID-19 national protocol is here! Also here is the COVID-19 PGD template. YAY we know what we are doing now. Legal mechanisms are all in-place for a busy Autumn booster campaign.
11th September: NEW COVID POSTER ALERT!!!!
10th September: COVID-19 training collection updated to refer to the National Minimum Standards and Core Curriculum for Vaccination Training (NMS) which includes guidance on COVID-19 vaccination. The COVID training standards and related competencies have now been withdrawn on the back of the new NMS (same for flu) so refer to that from now on for all your immunisation training standards and comps. Also updated for the autumn programme: COVID-19 vaccination: information for healthcare practitioners.
9th September: COVID-19 Green Book chapter updated with latest ordering information. Also updated is the Information for parents of eligible at-risk children aged 6 months to 11 years on COVID-19 vaccination.
5th September: COVID-19 vaccination: autumn programme resources- updated with information leaflet and sticker.
28th August: Ooooh look what's been updated! COVID-19: the green book chapter.
16th July: Departmental minute on the COVID-19 autumn 2025 vaccination programme AND 26/27 advice is here! Vaccination will be offered in England in autumn 2025 to adults aged 75 years and over, residents in a care home for older adults, individuals aged 6 months and over who are immunosuppressed, as defined in tables 3 and 4 of the COVID-19 Green Book chapter. The same groups look likely for autumn 2026 and spring 2027 too. The vaccines that will be supplied for the autumn 2025 programme are the Pfizer-BioNTech mRNA (Comirnaty) vaccines, dose-dependent on age.
I like this line from the June JCVI minutes: "Internationally, similarly low levels of COVID-19 activity had been seen across the winter. It was suggested that the stability of the recent epidemiology might reflect the slowing in viral evolution of SARS-CoV-2". Phew! COVID-19 seems to be losing momentum! (a bit like me towards the end of the school holidays). Another important thing mentioned in those minutes was that the two most recent vaccination campaigns had taken place after significant peaks in hospitalisations, as such indicating a possible misalignment of timing of vaccination with peaks in disease. HEADS UP: It was proposed that year-round vaccination, with individuals receiving a dose of COVID-19 vaccine twice a year, every six months, might be an alternative model for vaccine delivery. Benefits of a year-round delivery model were highlighted as;
Regular supply of vaccine which could change as a new product, such as an updated variant vaccine, became available
More outreach work could be undertaken to improve uptake in population groups with lower vaccine coverage throughout the year
Less disruption on routine healthcare services
The potential to vary or extend the interval between vaccination, depending on vaccine effectiveness estimates and cost-effectiveness analysis.
A year-round delivery model might facilitate more frequent vaccination for immunosuppressed individuals.
Well they are some good arguments, but they are undecided as of yet.
7th July 2025: New Covid strain spreads across UK with unique symptom. The XFG and XFG.3 variants currently account for around 30 percent of Covid-19 cases in England. Feeling hoarse? Could it be stratus?...
4th June: Cumulative uptake for the spring 2025 vaccination campaign, as of the 4th June 2025, was 55% in adults 75 years of age and over, and 23% in individuals under 75 years (including individuals who were immunosuppressed). Most campaigns plateaued in uptake between eight and twelve weeks after beginning.
6th Feb 2025: Updated resources: Safety of COVID-19 vaccines when given in pregnancy - Guidance for health professionals to share with pregnant women immunised with COVID-19 vaccines.
8th October 2024: Vaccine update landed! In there it highlighted that last year’s data shows that those who received a COVID-19 vaccine were around 45% less likely to be admitted to hospital with COVID-19, compared to those who did not receive one. But protection wanes 3 to 6 months following vaccination. That is why for those who are most vulnerable, we must keep going with those booster doses for the most vulnerable groups.

Reminders:
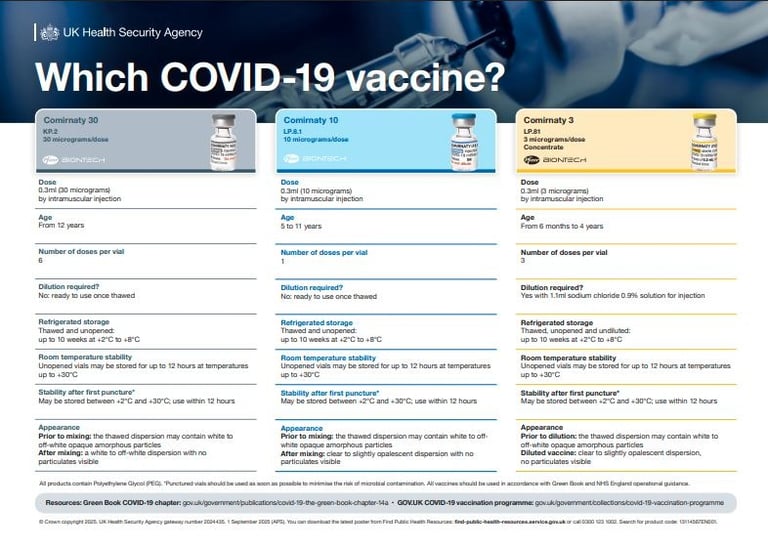
Current COVID-19 mRNA vaccines available in the UK:
Comirnaty LP.8.1 This is the Pfizer–BioNTech paediatric variant vaccine:
10 µg dose for children aged 5–11 years
3 µg dose for infants aged 6 months to 4 years
Comirnaty KP.2 A monovalent vaccine adapted to the KP.2 ("FLiRT") subvariant:
30 µg in pre‑filled syringe (and multidose vial) for individuals aged 12 and over
Comirnaty JN.1. A vaccine targeting the JN.1 subvariant, available in several age formulations:
30 µg for adults and children 12+
10 µg for children 5–11 years
3 µg for infants 6 months – 4 years
Spikevax JN.1 Moderna's adapted mRNA vaccine targeting JN.1 subvariant:
Approved for adults and children aged 6 months and over
Booster campaigns run autumn and spring (depending on age/risk)
Administered IM, usually deltoid, but those under 12m in the thigh
May be co-administered with flu vaccine now (the intervals have been withdrawn)
Store at 2–8°C after thawing (check SPC—shelf life varies by product)
Administer under PSD or PGD or National Protocol
Want to geek out? Go deeper here:
15th May 2025: If you want to learn more about the pipeline for COVID-19 vaccines and hear a REALLY GOOD explanation of all the different platforms and how to handle explaining this to the public then THIS IS THE (recording of a) WEBINAR FOR YOU! I really enjoyed it.
7th May 2025: I was wondering what the latest was on the combined flu and COVID vaccine, and then Fray very kindly sent me this: Moderna's combo Covid and flu mRNA shot outperforms current vaccines in large trial
5th Feb 2025: Nasal COVID-19 vaccine to enter US clinical trials. The new trial will evaluate the safety and efficacy of the vaccine administered via two routes: inhaled into the lungs and sprayed into the nose. I absolutely love seeing developments in vaccine administration technology.
Check out this intriguing headline: Administering the BCG vaccine during the active phase of COVID-19 may help protect against the development of long COVID.
SIREN study: The SIREN study has been investigating SARS-CoV-2 infections and acute respiratory illness in healthcare workers since 2020, and providing vital research into the immune response to infection and vaccination.
Covid infection may be prevented with a common nasal antihistamine spray
Lots about nasal spray at the minute: A common nasal spray may block Covid infection, trial results indicate. The over-the-counter antihistamine azelastine works against a range of respiratory infections, including flu and RSV, according to German researchers.
9th September 2025: Pfizer reports strong phase 3 clinical data for 2025-26 COVID vaccine
New study shows all-in-one coronavirus vaccines could save millions of lives in future pandemics
15th October 2025: Study finds no link between mRNA COVID vaccines early in pregnancy and birth defects



What Is COVID-19 Anyway?
COVID-19 is caused by the SARS-CoV-2 virus, a novel coronavirus first identified in 2019. It rapidly led to a global pandemic, causing widespread illness, disruption, and over 6 million deaths worldwide (WHO, 2024). While milder in many people today, COVID-19 still poses a significant risk to certain populations—particularly the elderly and immunocompromised.
What Happens If You Catch It?
COVID-19 affects people very differently. Symptoms can range from mild cold-like illness to life-threatening pneumonia, sepsis, and long COVID:
Common symptoms: cough, fever, fatigue, loss of taste or smell, headache, sore throat
Severe illness: difficulty breathing, chest pain, confusion, low oxygen levels
Long COVID: fatigue, brain fog, breathlessness, and other symptoms persisting for months
As of mid-2025, most severe cases occur in unvaccinated individuals or those with reduced immune response.
How Does It Spread?
Primarily via respiratory droplets and aerosols—talking, coughing, sneezing, and even just breathing in close quarters. Also spreads via contaminated hands and surfaces, especially in indoor or poorly ventilated environments.
Does Getting It Make You Immune?
Infection does provide some immunity, but this wanes over time, and new variants can bypass earlier protection. Vaccination boosts both infection and severe disease protection, especially against newer variants.
Can It Be Treated?
Yes—for higher-risk cases. Options include:
Antivirals: e.g. nirmatrelvir/ritonavir (Paxlovid®), remdesivir
Monoclonal antibodies: limited use as resistance patterns change
Supportive care: oxygen, fluids, anticoagulants, ICU if needed
Treatment is most effective when started early after symptom onset.
Who’s Most at Risk?
Adults aged 65+
People with chronic conditions (diabetes, heart disease, respiratory disease)
Immunocompromised individuals
Bits and bobs to casually drop into conversation
Did you know....
About 40% of people with COVID-19 have moderate symptoms and non-severe pneumonia, 15% have significant disease including severe pneumonia, and 5% experience critical disease with life-threatening complications.
So far, no-one has been able to identify the animal which led to human infection with the 2019 coronavirus -but there are reports that SARS-CoV-2 is most similar to that of bat coronaviruses.
The European Centre for Disease Control estimates that 1 in 1000 people die prematurely when infected with influenza. For COVID-19 the current estimates suggest that 10 in 1000 infected people will die from the disease in high-income countries. Handy statistic to know for those who say "I'll have my Flu jab but not that COVID-19 one".